Thermodynamic origin of dendrite growth in metal anode batteries†
Abstract
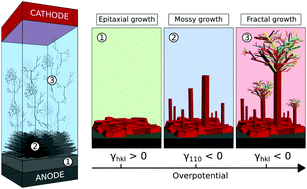
The formation of dendrites in alkali and alkaline earth-metal batteries leads to short circuit and catastrophic battery failure which hinders the development of high-energy-density battery technology. Herein, we investigate the thermodynamic origin of this complex phenomenon and show that kinetic transport limitation of metal cations in the electrolyte is not the only factor controlling the formation of dendrites. The specific behavior of Li, Na and Mg electrodes towards dendritic growth is straightforwardly deduced from the shape of their electro-capillary diagrams, as computed from a grand canonical DFT approach. The whisker and dendrite morphologies associated with the different growth regimes are fully rationalized by the present methodology and the critical parameters controlling the dendritic growth on metallic surfaces are clearly identified. Further improving the description of the interface by means of a simplified yet realistic SEI built on carbonate-based decomposition products, we show that the over-potentials at which each growth regime is expected to occur can be predicted at a quantitative level, hence allowing the design of chemical strategies to prevent dendrite growth at metallic surfaces. More specifically, high surface tension associated with low surface capacitance and low potential of zero-charge is the target triptych to favor safe battery operation and can be obtained through appropriate chemical engineering.


 Please wait while we load your content...
Please wait while we load your content...