Understanding the formation of bulk- and surface-active layered (oxy)hydroxides for water oxidation starting from a cobalt selenite precursor†
Abstract
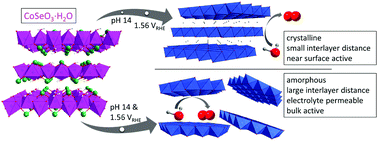
The urgent need for a stable, efficient, and affordable oxygen evolution reaction (OER) catalyst has led to the investigation of a vast amount of transition metal materials with multiple different anions. In situ and post catalytic characterization shows that most materials transform during the harsh OER conditions to layered (oxy)hydroxides (LOH). Several open questions concerning these in situ formed LOH remain such as: an explanation for their strongly varying activities, or the effect of the precatalyst structure, leaching anions, and transformation conditions on the formed LOH. Herein, we report on a cobalt selenite precursor, which, depending on pH and potential, transforms irreversibly into two different LOH OER catalysts. Combining multiple electrochemical and analytical methods ex and in situ, we prove that one of these products is near-surface catalytically active and the other one throughout the bulk with an in situ average cobalt oxidation state of 3.2. We deduce a detailed structural model explaining these differences and propose general concepts relating both the precatalyst structure and the transformation conditions to the final catalyst. Further, we apply these models to the most promising non-noble metal catalyst, NiFe LOH.


 Please wait while we load your content...
Please wait while we load your content...