Boosting alkaline hydrogen evolution: the dominating role of interior modification in surface electrocatalysis†
Abstract
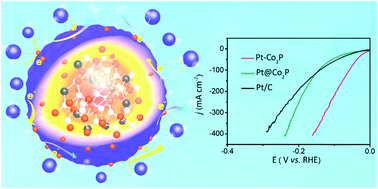
The alkaline hydrogen evolution reaction (A-HER) holds great promise for clean hydrogen fuel generation but its practical utilization is severely hindered by the sluggish kinetics for water dissociation in alkaline solutions. Traditional ways to improve the electrochemical kinetics for A-HER catalysts have been focusing on surface modification, which still can not meet the demanding requirements for practical water electrolysis because of catalyst surface deactivation. Herein, we report an interior modification strategy to significantly boost the A-HER performance. Specifically, a trace amount of Pt was doped in the interior Co2P (Pt–Co2P) to introduce a stronger dopant–host interaction than that of the surface-modified catalyst. Consequently, the local chemical state and electronic structure of the catalysts were adjusted to improve the electron mobility and reduce the energy barriers for hydrogen adsorption and H–H bond formation. As a proof-of-concept, the interior-modified Pt–Co2P shows a reduced onset potential at near-zero volts for the A-HER, low overpotentials of 2 mV and 58 mV to achieve 10 and 100 mA cm−2, and excellent durability for long-term utilization. The interior-modified Pt–Co2P delivers superior A-HER performance to Pt/C and other state-of-the-art electrocatalysts. This work will open a new avenue for A-HER catalyst design.


 Please wait while we load your content...
Please wait while we load your content...