Capturing the active sites of multimetallic (oxy)hydroxides for the oxygen evolution reaction†
Abstract
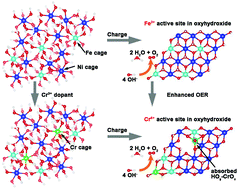
Efficient generation of H2via water-splitting is an underpinning technology for realizing the hydrogen economy. However, the sluggish anodic oxygen evolution reaction (OER) requires a large energy input. Low-cost, transition metals such as NiFe oxides/hydroxides have been regarded as one of the most efficient catalysts for the OER in alkaline media, although the detailed mechanisms remain debated due to the lack of direct evidence for the proposed active sites during the catalytic processes. Herein, we show a NiFe (oxy)hydroxide catalyst doped with a third metal Cr prepared by facile electrodeposition to achieve further enhanced activity for the OER. Operando Raman and X-ray absorption spectroscopy (XAS) characterisation were employed to detect the formation of active intermediates and M–O bonds on active sites during the OER process. For the host NiFe (oxy)hydroxide catalyst, the shorter Fe–O in the Fe-substituted-β-NiOOH intermediate is observed as active sites for the OER. A Cr, Fe-substituted-β-NiOOH intermediate is detected in the enhanced NiFeCr (oxy)hydroxide catalyst where Cr is oxidized into the 6+ valence state with optimal Cr–O bonds, adding new active sites to boost the OER. Density functional theory (DFT) calculations support the operando spectroscopic observations and reveal the lower overpotential at the Cr6+ sites in the NiFeCr oxyhydroxide intermediate than the Fe3+ sites in the NiFe oxyhydroxide intermediate. This study demonstrates a strategy for designing highly active OER catalysts by introducing high valence metals into oxides/hydroxides to further enhance the kinetics of water oxidation.


 Please wait while we load your content...
Please wait while we load your content...