Surface decoration accelerates the hydrogen evolution kinetics of a perovskite oxide in alkaline solution†
Abstract
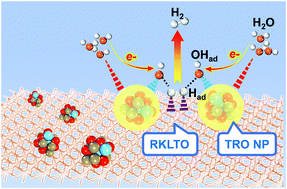
As an important class of inorganic compounds that exhibit a variety of physical, chemical, and electrochemical properties, ABO3±δ perovskites are generally known as active electrocatalysts for the anodic oxygen evolution reaction (OER). However, the inferior performance for the cathodic hydrogen evolution reaction (HER) limits their wide potential in constructing stable oxide-based alkaline electrolyzers for hydrogen production. Here, we show that the efficient decoration of perovskite oxide (K0.469La0.531)TiO3 (KLTO) via surface ion exchange with Ru cations and nucleation growth of Ti-doped RuO2 (TRO) nanoparticles could form a composite oxide-type electrocatalyst. It enables fast water dissociation with excellent HER activity in alkaline solution, superior to other oxide electrocatalysts and commercial Pt/C. Theoretical and experimental studies imply that the co-existence of surface TRO nanoparticles and the Ru-doped KLTO (RKLTO) substrate synergistically enhances the alkaline hydrogen evolution kinetics. Ti doping into the RuO2 lattice could significantly reduce the barrier of water dissociation to facilitate the Volmer process. The surface doping of Ru in the KLTO substrate could regulate and optimize the hydrogen adsorption free energy. The present strategy represents a new concept for designing oxide-based electrocatalysts related to devices for energy conversion and storage.


 Please wait while we load your content...
Please wait while we load your content...