Control of transition metal–oxygen bond strength boosts the redox ex-solution in a perovskite oxide surface†
Abstract
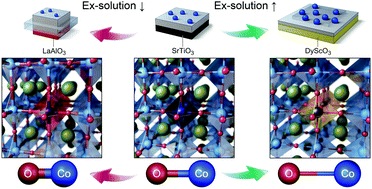
We demonstrate theoretically and experimentally that engineering of cation–oxygen bond strength in a perovskite structure can control redox ex-solution of B-site metals and thus the formation of metal nanoparticles at the oxide surface upon high-temperature reduction. In particular, we show that large isovalent doping significantly promotes the B-site ex-solution via tuning of the cation–oxygen bond strength, leading to high catalytic activity of CO oxidation. This method to promote ex-solution can be readily applied to various heterogeneous catalysts.
- This article is part of the themed collection: Energy & Environmental Science Cover Art


 Please wait while we load your content...
Please wait while we load your content...