Multiorbital bond formation for stable oxygen-redox reaction in battery electrodes†
Abstract
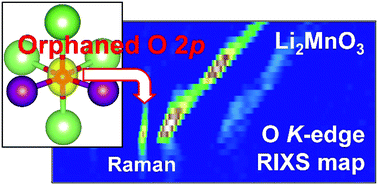
High-energy-density batteries have been a long-standing target toward sustainability, but the energy density of state-of-the-art lithium-ion batteries is limited in part by the small capacity of the positive electrode materials. Although employing the additional oxygen-redox reaction of Li-excess transition-metal oxides is an attractive approach to increase the capacity, an atomic-level understanding of the reaction mechanism has not been established so far. Here, using bulk-sensitive resonant inelastic X-ray scattering spectroscopy combined with ab initio computations, we demonstrate the presence of a localized oxygen 2p orbital weakly hybridized with transition metal t2g orbitals that was theoretically predicted to play a key role in oxygen-redox reactions. After oxygen oxidation, the hole in the oxygen 2p orbital is stabilized by the generation of either a (σ + π) multiorbital bond through strong π back-donation or peroxide O22− through oxygen dimerization. The multiorbital bond formation with σ-accepting and π-donating transition metals can thus lead to reversible oxygen-redox reaction.


 Please wait while we load your content...
Please wait while we load your content...