High-capacity thermochemical CO2 dissociation using iron-poor ferrites†
Abstract
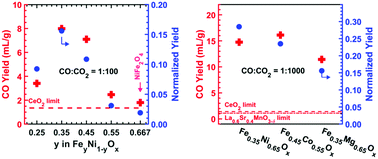
Dissociation of CO2 to form CO can play a key role in decarbonizing our energy system. We report here a two-step thermochemical cycle using a variety of iron-poor (Fe-poor) ferrites (FeyM1−yOx where y < 2/3) that produce CO with unusually high yield using Fe as the redox active species. Conventional wisdom suggests that increasing the Fe fraction would increase the capacity for CO2 dissociation. Here, we report the opposite result: at partial pressure ratio CO : CO2 = 1 : 100, we demonstrated CO yields of 8.0 ± 1.0 mL-CO per gram from Fe0.35Ni0.65Ox, and 3.7 ± 1.0 mL-CO per gram from Fe0.45Co0.55Ox, at a thermal reduction temperature of 1300 °C; remarkably, these CO2 dissociation capacities are significantly higher than those of state-of-the-art materials such as spinel ferrites (Fe2MO4), (substituted) ceria, and Mn-based perovskite oxides. Optimization of the kinetics of Fe-poor ferrites with a ZrO2 support resulted in higher CO yields per gram of ferrite. The unexpected CO yield vs. Fe ratio trend is consistent with the prediction of calculated ternary phase diagrams, which suggest a swing between spinel and rocksalt phases. These Fe-poor ferrites open new opportunities for tuning the redox properties of oxygen exchange materials.


 Please wait while we load your content...
Please wait while we load your content...