Electrical decoupling of microbial electrochemical reactions enables spontaneous H2 evolution†
Abstract
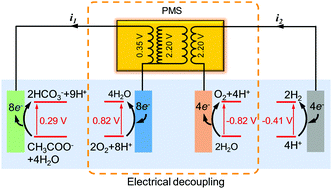
Hydrogen evolution is not a spontaneous reaction, so current electrochemical H2 systems either require an external power supply or use complex photocathodes. We present in this study that by using electrical decoupling, H2 can be produced spontaneously from wastewater. A power management system (PMS) circuit was deployed to decouple bioanode organic oxidation from abiotic cathode proton reduction in the same electrolyte. The special PMS consisted of a boost converter and an electromagnetic transformer, which harvested energy from the anode followed by voltage magnification from 0.35 V to 2.2–2.5 V, enabling in situ H2 evolution for over 96 h without consuming any external energy. This proof-of-concept demonstrated a cathode faradaic efficiency of 91.3% and a maximum overall H2 conversion efficiency of 28.9%. This approach allows true self-sustaining wastewater to H2 evolution, and the system performance can be improved via the PMS and reactor optimization.
- This article is part of the themed collection: Energy & Environmental Science Cover Art


 Please wait while we load your content...
Please wait while we load your content...