Phosphinoborane interception at magnesium by borane-assisted phosphine-borane dehydrogenation†
Abstract
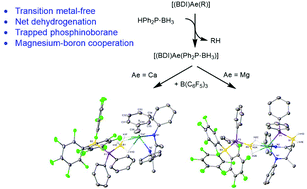
Reactions of B(C6F5)3 with the β-diketiminato (BDI) alkaline-earth phosphidoborane complexes, 1a [(BDI)Ca(H3B·PPh2)] and 1b [(BDI)Mg(H3B·PPh2)]2 (BDI = [HC{C(CH3)N(2,6-iPr-C6H3)}2]−) result in the formation of phosphinodiboronate complexes 4a [(BDI)Ca(η6-toluene){H3B·PPh2·B(C6F5)3}] and 4b [(BDI)Mg{H3B·PPh2·B(C6F5)3}]. Calcium complex 4a is stable in aromatic solvents at room temperature and does not display well-defined onward reactivity at elevated temperatures. Magnesium complex 4b undergoes a room temperature transformation to provide the known hydridoborate derivative 3b [(BDI)Mg{HB(C6F5)3}] and an N,P,N’-ligated species, 5 [{HC(C(CH3)N(2,6-iPr-C6H3))2(H2BPPh2)}Mg{H3B·PPh2·B(C6F5)3}] that results from interception of the putative phosphinoborane, H2B = PPh2, by the BDI ligand backbone following B(C6F5)3-mediated hydride abstraction. NMR spectroscopic investigations were supported by DFT calculations, which suggested a mechanism involving B(C6F5)3 migration and hydride abstraction within the coordination sphere of magnesium. Interception of H2B = PPh2 by B(C6F5)3 is proposed to stabilise this species, whilst activating it towards ligand-centred nucleophilic attack. The significant stabilisation energy calculated for the Ca-π(toluene) interaction in 4a accounts for the contrasting outcomes between the two Ae-elements. The crystal structures of compounds 4a and 5 are presented and discussed.


 Please wait while we load your content...
Please wait while we load your content...