Palladium complexes with an annellated mesoionic carbene (MIC) ligand: catalytic sequential Sonogashira coupling/cyclization reaction for one-pot synthesis of benzofuran, indole, isocoumarin and isoquinolone derivatives†
Abstract
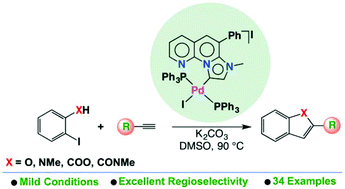
Two Pd(II) complexes (1 and 2) featuring a fused π-conjugated imidazo[1,2-a][1,8]naphthyridine-based mesoionic carbene ligand have been synthesized and structurally characterized. Both complexes effectively catalyze the one-pot synthesis of benzofuran starting from phenylacetylene and 2-iodophenol under mild conditions. Complex 1 is found to be an excellent catalyst for the straightforward access to a library of benzofuran, indole, isocoumarin and isoquinolone derivatives by the reaction of terminal alkynes with 2-iodo derivates of phenol, N-methyl aniline, benzoic acid and N-methyl benzamide, respectively. The general utility of the catalytic method is demonstrated using a variety of diversely substituted terminal alkynes and the corresponding desired products are obtained in good to excellent yields. On the basis of control experiments, a two-cycle mechanism is proposed which involves the Sonogashira coupling of 2-iodo derivatives with alkynes and the subsequent cyclization of the corresponding 2-alkynyl compounds.


 Please wait while we load your content...
Please wait while we load your content...