Electronic structures and bonding of the actinide halides An(TRENTIPS)X (An = Th–Pu; X = F–I): a theoretical perspective†
Abstract
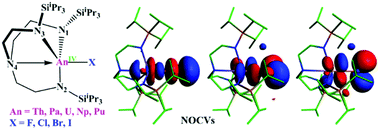
To evaluate how halogen and actinide atoms affect the electronic structures and bonding nature, we have theoretically investigated a series of the actinide halides An(TRENTIPS)X (An = Th–Pu; X = F–I); several of them have been synthesized by Liddle's group. The An–X bond distances decrease from An = Th to Pu for the same halides, and the harmonic vibrational frequencies for the An–X bonds are more susceptible to being affected by the halogen atoms. The analyses of bonding nature reveal that the An–X bonds have a certain covalency with a polarized character, and the σ-bonding component in the total orbital contribution is greatly larger than the corresponding π-bonding ones based on the analysis of the NOCVs (the natural orbitals for chemical valence). Furthermore, the electronic structures of the thorium complexes are obviously different from those of the uranium and transuranic analogues due to more valence electrons in Th 6d orbitals. In addition, thermodynamic results suggest that the U(TRENTIPS)Br complex is the most stable and U(TRENTIPS)Cl has the highest reactivity based on the halide exchange reaction of U(TRENTIPS)X complexes using Me3SiX. The reduction ability of the tetravalent An(TRENTIPS)X is sensitive to halogen atoms according to the calculated electron affinity of the An(TRENTIPS)X and the reactions An(TRENTIPS)X + K → An(TRENTIPS) + KX. This work presents the effect of the halogen and the actinide atoms on the structures, bonding nature and redox ability of a series of the tetravalent actinide halides with TREN ligand and facilitates our in-depth understanding of f-block elements, which could provide theoretical guidance for experimental work on actinide halides, especially for the synthetic chemistry of transuranic halides.


 Please wait while we load your content...
Please wait while we load your content...