Nopinone-based AIE-active dual-functional fluorescent chemosensor for Hg2+ and Cu2+ and its environmental and biological applications†
Abstract
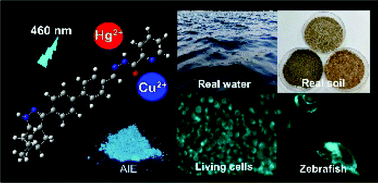
A highly sensitive AIE fluorescent chemosensor, N′-((4′-(6,6-dimethyl-4,5,6,7-tetrahydro-1H-5,7-methanoindazol-3-yl)-[1,1′-biphenyl]-4-yl)methylene)picolinohydrazide (PBPHS), for the detection of Hg2+ and Cu2+ ions in near 100% aqueous solution was developed based on the renewable β-pinene derivative nopinone. The probe PBPHS was designed and synthesized via a four reaction steps, including condensation, cyclization, Suzuki coupling reaction, and Schiff-base reaction. PBPHS could recognize Hg2+ and Cu2+ over other competitive metal ions via specific complexation ability towards them. The detection limits of PBPHS for Hg2+ and Cu2+ were 15 nM and 17 nM, respectively. The fluorescent probe PBPHS could also be utilized to detect S2− and Hg2+/Cu2+ reversibly for five cycle. Moreover, the different binding mechanisms of PBPHS with Hg2+ and Cu2+ were confirmed by HRMS analysis, 1H NMR titration, DFT calculation, and molecular logic gate. Furthermore, the probe PBPHS was applied for the analysis of real water and soil samples and living HeLa cells and zebrafish bioimaging.


 Please wait while we load your content...
Please wait while we load your content...