Three types of copper derivatives formed by CuCl2·2H2O interaction with (Z)-3-aryl-2-(methylthio)-5-(pyridine-2-ylmethylene)-3,5-dihydro-4H-imidazol-4-ones†
Abstract
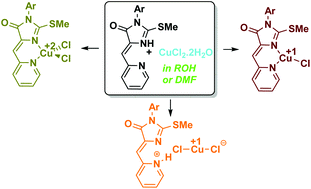
The reactions of (Z)-3-aryl-2-(methylthio)-5-(pyridine-2-ylmethylene)-3,5-dihydro-4H-imidazol-4-ones (3) with CuCl2·2H2O in the presence of a reducing solvent (alcohol or dimethylformamide (DMF)) produce three types of Cu-containing compounds: two Cu complexes with a composition of CuII(3)Cl2 (4) and CuI(3)Cl (5) as well as a salt (3 + H)+CuICl2− (6) in a 4 : 5 : 6 ratio depending on the substituent at the N(3) nitrogen atom of the ligand moiety. In non-reducing solvents (dimethyl sulfoxide (DMSO) and CHCl3/acetone), only complexes 4 were formed, All three Cu derivatives (4, 5, and 6) were characterized by single-crystal X-ray diffraction, UV/vis spectroscopy, and electrochemistry data. Convenient electrochemical and UV-vis spectral criteria were recorded, which made it possible to distinguish between the different Cu-containing compounds. Based on the electron spectroscopy and electron paramagnetic resonance (EPR) data, a possible scheme for the formation of compounds 4–6 was proposed, including the initial coordination of copper(II) chloride with an organic ligand, the subsequent reduction of the resulting complex 4 by DMF with the formation of salt 6, and the further transition of salt 6 into the complex 5.


 Please wait while we load your content...
Please wait while we load your content...