Cytotoxic (cis,cis-1,3,5-triaminocyclohexane)ruthenium(ii)-diphosphine complexes; evidence for covalent binding and intercalation with DNA†
Abstract
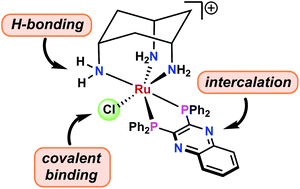
We report cytotoxic ruthenium(II) complexes of the general formula [RuCl(cis-tach)(diphosphine)]+ (cis-tach = cis–cis-1,3,5-triaminocyclohexane) that have been characterised by 1H, 13C and 31P{1H} NMR spectroscopy, mass spectrometry, X-ray crystallography and elemental analysis. The kinetics of aquation and stability of the active species have been studied, showing that the chlorido ligand is substituted by water at 298 K with first order rate constants of 10−2–10−3 s−1, ideal for potential clinical use as anti-tumour agents. Strong interactions with biologically relevant duplex and quadruplex DNA models correlate with the activity observed with A549, A2780 and 293T cell lines, and the degree of activity was found to be sensitive to the chelating diphosphine ligand. A label-free ptychographic cell imaging technique recorded cell death processes over 4 days. The Ru(II) cis-tach diphosphine complexes exhibit anti-proliferative effects, in some cases outperforming cisplatin and other cytotoxic ruthenium complexes.


 Please wait while we load your content...
Please wait while we load your content...