Experimental study of speciation and mechanistic implications when using chelating ligands in aryl-alkynyl Stille coupling†‡
Abstract
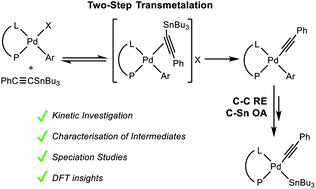
Neutral palladium(II) complexes [Pd(Rf)X(P–L)] (Rf = 3,5-C6Cl2F3, X = Cl, I, OTf) with P–P (dppe and dppf) and P–N (PPh2(bzN)) ligands have chelated structures in the solid-state, except for P–L = dppf and X = Cl, were chelated and dimeric bridged structures are found. The species present in solution in different solvents (CDCl3, THF, NMP and HMPA) have been characterised by 19F and 31P{1H} NMR and conductivity studies. Some [Pd(Rf)X(P–L)] complexes are involved in equilibria with [Pd(Rf)(solv)(P–L)]X, depending on the solvent and X. The ΔH° and ΔS° values of these equilibria explain the variations of ionic vs. neutral complexes in the range 183–293 K. Overall the order of coordination strength of solvents and anionic ligands is: HMPA ≫ NMP > THF and I−, Cl− > TfO−. This coordination preference is determining the complexes participating in the alkynyl transmetalation from PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CSnBu3 to [Pd(Rf)X(P–L)] (X = OTf, I) in THF and subsequent coupling. Very different reaction rates and stability of intermediates are observed for similar complexes, revealing neglected complexities that catalytic cycles have to deal with. Rich information on the evolution of these Stille systems after transmetalation has been obtained that leads to proposal of a common behaviour for complexes with dppe and PPh2(bzN), but a different evolution for the complexes with dppf: this difference leads the latter to produce PhC
CSnBu3 to [Pd(Rf)X(P–L)] (X = OTf, I) in THF and subsequent coupling. Very different reaction rates and stability of intermediates are observed for similar complexes, revealing neglected complexities that catalytic cycles have to deal with. Rich information on the evolution of these Stille systems after transmetalation has been obtained that leads to proposal of a common behaviour for complexes with dppe and PPh2(bzN), but a different evolution for the complexes with dppf: this difference leads the latter to produce PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CRf and black Pd, whereas the two former yield PhC
CRf and black Pd, whereas the two former yield PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CRf and [Pd(C
CRf and [Pd(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPh)(SnBu3)(dppe)] or [Pd(C
CPh)(SnBu3)(dppe)] or [Pd(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPh)(SnBu3){PPh2(bzN)}].
CPh)(SnBu3){PPh2(bzN)}].


 Please wait while we load your content...
Please wait while we load your content...