Potent cyclometallated Pd(ii) antitumor complexes bearing α-amino acids: synthesis, structural characterization, DNA/BSA binding, cytotoxicity and molecular dynamics simulation†
Abstract
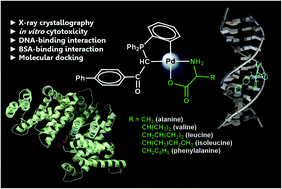
A rational approach was adopted to design high-potential metal-based antitumor agents. A series of organometallic Pd(II) complexes with a general formula of [Pd{κ2(C,C)-[(C6H4-2)PPh2]CH(CO)C6H4Ph-4}{κ2(N,O)}] (N,O = alanine (Pd-A), valine (Pd-V), leucine (Pd-L), L-isoleucine (Pd-I) and phenylalanine (Pd-F)) were prepared by cyclopalladation of the phosphorus ylide, bridge cleavage reaction and subsequent chelation of natural α-amino acids. The complexes were fully identified using IR and multinuclear 1H, 13C, 31P NMR spectroscopic methods. X-ray crystallography exhibited that the Pd(II) atom is located in a slightly distorted square-planar environment surrounded by C,C-orthometallated phosphorus ylide as well as NO-pendant amino acid functionality. In vitro cytotoxicity evaluation of new cyclometallated Pd(II) complexes toward a human leukemia (K562) cancer cell line indicated promising results. The highest cytotoxic activity was discovered in the case of phenylalanine (CH2C6H5). IC50 values of this complex on a panel of human tumor cell lines representative of liver (HepG2), breast (SKBR-3), and ovarian (A2780-Resistance/Sensitive) cancers also indicated promising antitumor effects in comparison with standard cisplatin. The binding interaction ability of the phenylalanine-containing orthopalladated complex, as the most efficient compound, with calf-thymus deoxyribonucleic acid (CT-DNA) and bovine serum albumin (BSA) was investigated. UV-Vis spectroscopy, competitive emission titration, and circular dichroism (CD) techniques demonstrated the intercalative binding of the Pd(II) complex with DNA. Molecular docking studies also fully agreed with the experimental data. Examination of the reactivity towards the protein BSA revealed that the static quenching mechanism of BSA intrinsic fluorescence by the Pd(II) complex with a binding constant (Kb) of ∼105 is indicative of the high affinity of the complex. The competitive binding experiment using site markers with definite binding sites demonstrated that the hydrophobic cavities of site I (subdomain IIA) are responsible for the bimolecular interaction between protein BSA and the complex. Molecular docking studies effectively confirmed the significance of hydrophobic interactions in Pd(II)–BSA binding. The results of this study could greatly contribute to exploring new potent metal-based anticancer drugs.


 Please wait while we load your content...
Please wait while we load your content...