Transfer hydrogenation of aldehydes and ketones catalyzed using an aminophosphinite POCNH pincer complex of Ni(ii)†
Abstract
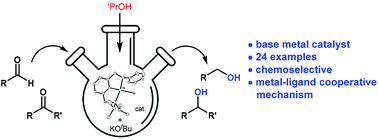
The aminophosphinite pincer complex (POCNH)NiBr was found to effectively catalyze the transfer hydrogenation of aldehydes and ketones with 2-propanol and KOtBu as a base, presenting a rare example of bifunctional nickel transfer hydrogenation catalysts. The transfer hydrogenation of aldehydes and ketones was found to be selective, tolerating a wide range of other functional groups, including those prone to reduction, such as esters, amides, alkenes, pyridines, and nitriles. The reactions were suggested to proceed via the metal–ligand cooperative mechanism with an intermediacy of an amido (POCN)NiII species.


 Please wait while we load your content...
Please wait while we load your content...