Synthesis, UV-visible spectroelectrochemistry and theoretical characterization of new polypyridyl Ru(ii) complexes containing 2,4,6-tris(2-pyridyl)-1,3,5-triazine as precursors for water oxidation catalysts†
Abstract
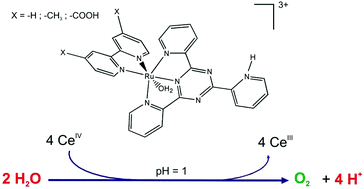
In this work, we report the syntheses and physicochemical characterization of new chloro and aqua complexes of Ru(II) with 2,4,6-tris(2-pyridyl)-1,3,5-triazine (tptz) and 2,2′-bipyridines substituted with donor and acceptor groups in the 4,4′-positions. The aqua complexes behave as precursors for water oxidation catalysts at pH = 1 using Ce(IV) as a sacrificial oxidant. Besides, the oxidized forms Ru(IV) and Ru(V) have been characterized at different pH values by electrochemistry, UV-Visible spectroscopy and spectroelectrochemistry. The reaction mechanisms were studied by combining mixing and stopped-flow experiments with spectrophotometric monitoring in the UV-visible region and all the rate constants were determined together with the corresponding TON and TOF values at pH = 1. Calculations based on Density Functional Theory (DFT and TD-DFT) were performed to support the experimental data.


 Please wait while we load your content...
Please wait while we load your content...