Detailed characterization of dioxouranium(vi) complexes with a symmetrical tetradentate N2O2-benzil bis(isonicotinoyl hydrazone) ligand†
Abstract
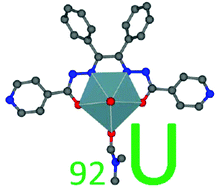
The reactions of UO2(OAc)2·2H2O with benzil bis(isonicotinoyl hydrazone) ligand (H2L) in varied solvent media resulted in the formation of a series of new dioxouranium(VI) complexes 1–3 of the type UO2(L)(X), [where 1, X = DMF; 2, X = DMSO; 3, X = H2O]. The complexes were systematically characterized by elemental analysis, UV-Visible spectroscopy, TGA, mass spectrometry, cyclic voltammetry, and powder X-ray diffraction study. Among all the complexes, 1 was confirmed by single-crystal X-ray diffraction study. It was found that 1 preferred a distorted pentagonal bipyramidal geometry, in which an equatorial coordination plane was formed by the ONNO-tetradentate cavity of the deprotonated hydrazone ligand along with an additional oxygen atom of the coordinated solvent molecule. Thermal analysis suggested that complexes 1 and 3 undergo weight loss in the temperature range 180–210 °C and 100–120 °C, respectively, due to the ready release of their coordinated solvent molecules. Complexes 1–3 exhibited analogous UV-Visible absorption bands and the intense band between 300–600 nm was assigned to the M ← L and n → π* transitions. Weakly resolved reduction waves assigned to {UO2}2+/{UO2}+ couple were observed for complexes 1 and 2 {1, −1.76 V; 2, −1.75 V; vs. ferrocenium/ferrocene (Fc+/Fc)} in DMSO solution, signifying the feeble electron-donating nature of the L2− ligand. Powder X-ray diffraction study suggested that the crystallite size of all the complexes was in the nanoscale range. Further analysis using density functional theory (DFT) calculations provided structural insights as well as information on the electronic properties of both complex 1 and the ligand.


 Please wait while we load your content...
Please wait while we load your content...