Electronic effect of a perfluorinated β-diketiminate ligand on the bonding nature of copper carbonyl complexes†
Abstract
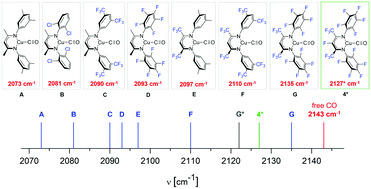
Two copper complexes 17Fnac2Cu(C6H6) (3) and 17Fnac2CuCO (4) containing the monoanionic, perfluorinated β-diketiminate 17Fnac2− ligand (1) (17Fnac2 = FC[C(CF3)N(C6F5)]2) were synthesized and characterized by IR and NMR spectroscopy (1H, 13C, 19F), cyclovaltammometry (CV), elemental analysis and single crystal X-ray diffraction. The perfluorinated 17Fnac2− ligand marginally reduces the π-back-bonding capacity of the copper centre to the carbonyl group in 4 when compared with the corresponding 16Fnac2− substituted complexes but substantially when compared with the fluorine free substituted derivatives. Quantum chemical calculations gave deeper insight into the bonding situation of this carbonyl complex, while CV studies were performed to determine the oxidation potential of 3 in solution. Based on these data, the influence of the degree of fluorination in different β-diketimine ligands on the electronic nature of the corresponding copper complexes is discussed.
- This article is part of the themed collection: Spotlight Collection: Fluorinated ligands


 Please wait while we load your content...
Please wait while we load your content...