Kinetic studies on the reaction of NO with iron(ii) complexes using low temperature stopped-flow techniques†‡
Abstract
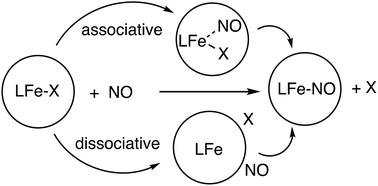
Low temperature stopped-flow techniques were used to investigate the reaction of three different iron(II) complexes with nitrogen monoxide. The kinetic studies allowed calculation of the activation parameters from the corresponding Eyring plots for all three systems. The reaction of iron(II) chloride with NO leading to the formation of MNIC (mononitrosyl-iron-complex) and DNIC (dinitrosyl-iron-complex) led to activation parameters of ΔH‡ = 55.4 ± 0.4 kJ mol−1 and ΔS‡ = 13 ± 2 J K−1 mol−1 for MNIC and ΔH‡ = 32 ± 6 kJ mol−1 and ΔS‡ = −193 ± 21 J K−1 mol−1 for DNIC. Formation of MNIC turned out to be much faster in comparison with DNIC. In contrast, activation parameters for the formation of monoculear [Fe(bztpen)(NO)](OTf)2 (bztpen = N-benzyl-N,N′,N′-tris(2-pyridylmethyl)-ethylenediamine) ΔH‡ = 17.8 ± 0.8 kJ mol−1 and ΔS‡ = −181 ± 3 J K−1 mol−1 supported an associative mechanism. Interestingly, [Fe(bztpen)(CH3CN)](OTf)2 does not react with dioxygen at all. Furthermore, activation parameters of ΔH‡ = 37.7 ± 0.7 kJ mol−1 and ΔS‡ = −66 ± 3 J K−1 mol−1 were obtained for the reaction of NO with the dinuclear iron(II) H-HPTB complex (H-HPTB = N,N,N′,N′-tetrakis(2-benzimidazolylmethyl)-2-hydroxy-1,3-diaminopropane), [Fe2(H-HPTB)(Cl)3]. The kinetic data allowed postulation of the mechanisms for all of these reactions.
- This article is part of the themed collection: Inorganic Reaction Mechanisms


 Please wait while we load your content...
Please wait while we load your content...