Ciprofloxacin conjugated to diphenyltin(iv): a novel formulation with enhanced antimicrobial activity†
Abstract
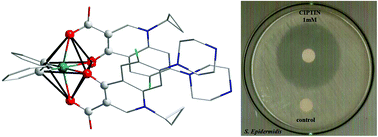
The metalloantibiotic of formula Ph2Sn(CIP)2 (CIPTIN) (HCIP = ciprofloxacin) was synthesized by reacting ciprofloxacin hydrochloride (HCIP·HCl) (an antibiotic in clinical use) with diphenyltin dichloride (Ph2SnCl2DPTD). The complex was characterized in the solid state by melting point, FT-IR, X-ray Powder Diffraction (XRPD) analysis, 119Sn Mössbauer spectroscopy, X-ray Fluorescence (XRF) spectroscopy, and Thermogravimetry/Differential Thermal Analysis (TG-DTA) and in solution by UV-Vis, 1H NMR spectroscopic techniques and Electrospray Ionisation Mass Spectrometry (ESI-MS). The crystal structure of CIPTIN and its processor HCIP was also determined by X-ray crystallography.
The antibacterial activity of CIPTIN, HCIP·HCl, HCIP and DPTD was evaluated against the bacterial species Pseudomonas aeruginosa (P. aeruginosa), Escherichia coli (E. coli), Staphylococcus aureus (S. aureus) and Staphylococcus epidermidis (S. epidermidis), by the means of Minimum Inhibitory Concentration (MIC), Minimum Bactericidal Concentration (MBC) and Inhibition Zones (IZs). CIPTIN shows lower MIC values than those of HCIP·HCl (up to 4.2-fold), HCIP (up to 2.7-fold) or DPTD (>135-fold), towards the tested microbes. CIPTIN is classified into bactericidal agents according to MBC/MIC values. The developing IZs are 40.8 ± 1.5, 34.0 ± 0.8, 36.0 ± 1.1 and 42.7 ± 0.8 mm, respectively which classify the microbes P. aeruginosa, E. coli, S. aureus and S. epidermidis to susceptible ones to CIPTIN. These IZs are greater than the corresponding ones of HCIP·HCl by 1.1 to 1.5-fold against both the tested Gram negative and Gram positive bacteria. CIPTIN eradicates the biofilm of P. aeruginosa and S. aureus more efficiently than HCIP·HCl and HCIP. The in vitro toxicity and genotoxicity of CIPTIN were tested against human skin keratinocyte cells (HaCaT) (IC50 = 2.33 μM). CIPTIN exhibits 2 to 9-fold lower MIC values than its IC50 against HaCaT, while its genotoxic effect determined by micronucleus assay is equivalent to the corresponding ones of HCIP·HCl or HCIP.


 Please wait while we load your content...
Please wait while we load your content...