Designing asymmetric Dy2 single-molecule magnets with two-step relaxation processes by the modification of the coordination environments of Dy(iii) ions†
Abstract
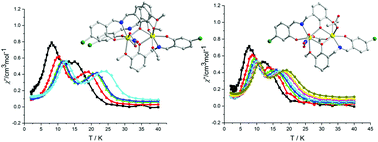
The reaction of Dy(NO3)3·6H2O and an asymmetric Schiff-base linker 5-chloro-2-(((2-hydroxy-3-methoxybenzyl)imino)methyl) phenol (H2L) afforded a dinuclear compound [Dy2L2(HL)(NO3)(EtOH)]·0.5C2H5OH (1). Complex 1 features two inequivalent Dy(III) sites, where three ligand sets (one HL− moiety and two L2− groups) are shared by two Dy(III) ions. The strategic introduction of CH3COOH in the reaction system used for synthesizing 1 induces the replacement of the HL− ligand by the CH3COO− ion, consequently resulting in the generation of [Et3NH][Dy2L2(NO3)(CH3COO)2] (2). In complex 2, two Dy(III) centers adopt NO7 (D2d geometry) and NO6 (C2v) coordination sphere, respectively. DC magnetic susceptibility studies for the two complexes indicate ferromagnetic interactions. Complexes 1 and 2 exhibit single-molecule magnet behavior with two-step slow relaxation processes due to the possession of inequivalent metal sites. The energy barriers of 69.19 and 45.73 K for 1, and 92.77 and 72.95 K for 2 are determined. Theoretical calculations reveal that the two-step relaxation processes in both 1 and 2 mainly originate from the single-ion magnetism of two distinct Dy(III) ions with inequivalent coordination environments.


 Please wait while we load your content...
Please wait while we load your content...