Oxidation reactions of a versatile, two-coordinate, acyclic iminosiloxysilylene†
Abstract
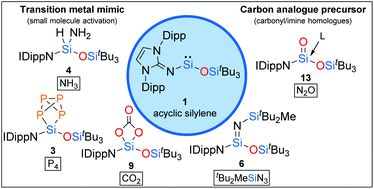
Due to their outstanding reactivity, acyclic silylenes have emerged as attractive organosilicon alternatives for transition metal complexes on the way to metal-free catalysis. However, exploration of their reactivity is still in its infancy, as only a few derivatives of this unique compound class have been isolated so far. Here, we present the results of an extensive reactivity investigation of the previously reported acyclic iminosiloxysilylene 1. Divalent silylene 1 proved to be a versatile building block for a plethora of novel organosilicon compounds. Thus, not only the activation of the rather challenging targets NH3 and P4 could be achieved, but also the conversion into a reactive donor-free silaimine, which itself turned out to be a useful reagent for small molecule activation. In addition, 1 served as an excellent precursor for gaining access to donor-stabilized heavier carbonyl compounds. Our results thus provide further insights into the chemistry of low-valent silicon at the interface between carbon and transition metals.


 Please wait while we load your content...
Please wait while we load your content...