Luminescent Pt(2,6-bis(N-methylbenzimidazol-2-yl)pyridine)X+: a comparison with the spectroscopic and electrochemical properties of Pt(tpy)X+ (X = Cl, CCPh, Ph, or CH3)†
Abstract
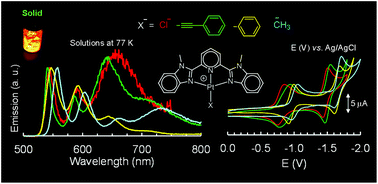
A series of platinum(II) pincer complexes of the formula Pt(mbzimpy)X+, 1(a–d), (mbzimpy = 2,6-bis(N-methylbenzimidazol-2-yl)pyridine; X = Cl; (a), CCPh; (b), Ph; (c), or CH3; (d), CCPh = phenylacetylide, and Ph = Phenyl) have been synthesized and characterized. Electronic absorption and emission, as well as electrochemical properties of these compounds, have been investigated. Pt(tpy)X+ analogs (tpy = 2,2′;6′2′′-terpyridine), 2(a–d), have also been investigated and compared. Electrochemistry shows that 1 and 2 analogs undergo two chemically reversible one-electron reduction processes that are shifted cathodically along the a < b < c < d series. Notably, these reductions occur at slightly higher negative potentials in the case of 1. The absorption spectra of 1 and 2 in acetonitrile exhibit ligand-centered (1LC) transitions (ε ≈ 104 M−1 cm−1) in the UV region and metal-to-ligand-charge transfer (1MLCT) transitions (ε ≈ 103 M−1 cm−1) in the visible region. The corresponding visible bands of 1b and 2b have been assigned to 1(LLCT/MLCT) mixed state (LLCT: ligand-to-ligand-charge transfer). The preceding 1LC and 1MLCT transitions of 1 occur at lower energies than that of 2. These 1LC transitions have distinctly been blue-shifted along a < c < d in 2, but occur at nearly identical energies in 1. Conversely, 1MLCT transitions are red-shifted along a < c < d in both the analogs. The 77 K glassy solutions of 1 and 2 exhibit an intense vibronically-structured emission band at λmax(0–0) in the 470–560 nm range. This band is red-shifted along b < a ≤ c < d in 1 and along a ≤ d ≈ c ≪ b in 2. The main character of these emissions is assigned to 3LLCT emissive state in 1b and 2b, whereas to 3LC in the rest of the compounds. Relative stabilization of these spin-forbidden emissive states is discussed by invoking configuration mixing with the higher-lying 3MLCT state.


 Please wait while we load your content...
Please wait while we load your content...