Halogenated isophthalamides and dipicolineamides: the role of the halogen substituents in the anion binding properties†
Abstract
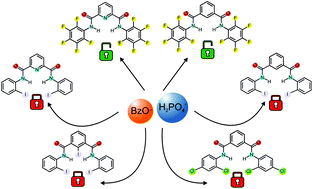
A novel family of amide-based receptors is herein described. Specifically, the role of the halogen substituents at the aryl moieties in the anion binding properties of a series of halogenated isophthalamides and dipicolineamides (L1–L6) was investigated both in solution and in the solid state in order to evaluate the incidence of all possible different and combined weak host–guest interactions. Only L5 and L6 bearing pentafluorophenyl rings as substituents have some affinities for the set of anions studied. In particular, in the case of L5 an interesting behaviour with the formation of a non-symmetric adduct with benzoate and dihydrogen phosphate was hypothesised by 1H- and 19F-NMR spectroscopy studies in solution and confirmed by theoretical calculation. The study of the crystal structures of the receptors demonstrated that the steric hindrance determined by the halogen substituents in the receptor molecules influences the accessibility of the anions to the isophthalamide or dipicoline amide NH moieties, thus modulating the affinity for the anion guests.


 Please wait while we load your content...
Please wait while we load your content...