Abstract
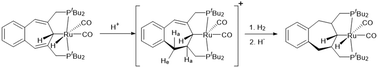
The benzo annulated cycloheptatriene PCP pincer ruthenium hydrido dicarbonyl complex syn-2 was prepared in one step by treatment of the ligand 1 with Ru3(CO)12. Protonation of syn-2 with the superacid [H(Et2O)2][BArF24] {[BArF24]− = tetrakis[bis(trifluoromethyl)phenyl]borate} initiates the highly stereoselective hydrogenation of one of the double bonds in the cycloheptatriene backbone. This results in the formation of the pentacoordinate cationic 16-electron dicarbonyl ruthenium complex 3. Hydrogenation of 3 with LiAlH4 generates the hydride complexes syn-4 and anti-4 which after protonation allow isolation of the symmetric 5. In 5 a second double bond of the cycloheptatriene backbone was hydrogenated. Complex 5 was also obtained directly by the reaction of 3 with hydrogen (1 bar). Storage of 5 under a hydrogen atmosphere yields two pairs of η2-H2 complexes (syn-7, anti-7) which are in a tautomeric equilibrium with their corresponding dihydrides (syn-8, anti-8). A stepwise transfer of the hydrides to the ligand backbone can be deduced from the distribution of deuterium along the seven membered ring after applying the deuterated reagents (D+, D2). The observed stereochemistry suggests that the hydride transfer is controlled by conformational constraints.


 Please wait while we load your content...
Please wait while we load your content...