Benzenedithiolate-bridged MoFe complexes: structures, oxidation states, and reactivities†
Abstract
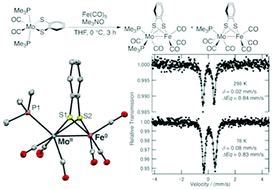
Two benzendithiolate-bridged MoFe complexes, [(Me3P)2(CO)2Mo(μ-S2C6H4)Fe(CO)3] (1) and [(Me3P)(CO)3Mo(μ-S2C6H4)Fe(CO)3] (2), were synthesized by reacting [Mo(S2C6H4)(CO)2(PMe3)2] (3) with Fe(CO)5. Each complex has a direct Mo–Fe bond that is supported by a bridging benzenedithiolate ligand and a semi-bridging carbonyl ligand as elucidated by single-crystal X-ray diffractometry. The structural data and differences in reactivity of these complexes suggest that monophosphine complex 2 is formed via diphosphine complex 1. The reaction of 2 with PMe3 gives the diiron bis(dithiolate) complex, [Fe(S2C6H4)(CO)2(PMe3)]2 (4), rather than 1. 57Fe Mössbauer and X-ray photoelectron spectroscopy studies reveal the oxidation states of the metal centers in 2 to be Fe0 and MoII.


 Please wait while we load your content...
Please wait while we load your content...