Robust Mn(iii) N-pyridylporphyrin-based biomimetic catalysts for hydrocarbon oxidations: heterogenization on non-functionalized silica gel versus chloropropyl-functionalized silica gel†
Abstract
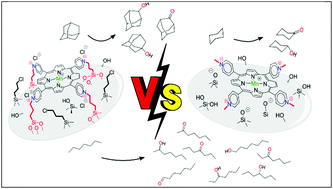
Two classes of heterogenized biomimetic catalysts were prepared and characterized for hydrocarbon oxidations: (1) by covalent anchorage of the three Mn(III) meso-tetrakis(2-, 3-, or 4-pyridyl)porphyrin isomers by in situ alkylation with chloropropyl-functionalized silica gel (Sil-Cl) to yield Sil-Cl/MnPY (Y = 1, 2, 3) materials, and (2) by electrostatic immobilization of the three Mn(III) meso-tetrakis(N-methylpyridinium-2, 3, or 4-yl)porphyrin isomers (MnPY, Y = 4, 5, 6) on non-modified silica gel (SiO2) to yield SiO2/MnPY (Y = 4, 5, 6) materials. Silica gel used was of column chromatography grade and Mn porphyrin loadings were deliberately kept at a low level (0.3% w/w). These resulting materials were explored as catalysts for iodosylbenzene (PhIO) oxidation of cyclohexane, n-heptane, and adamantane to yield the corresponding alcohols and ketones; the oxidation of cyclohexanol to cyclohexanone was also investigated. The heterogenized catalysts exhibited higher efficiency and selectivity than the corresponding Mn porphyrins under homogeneous conditions. Recycling studies were consistent with low leaching/destruction of the supported Mn porphyrins. The Sil–Cl/MnPY catalysts were more efficient and more selective than SiO2/MnPY ones; alcohol selectivity may be associated with hydrophobic silica surface modification reminiscent of biological cytochrome P450 oxidations. The use of widespread, column chromatography, amorphous silica yielded Sil-Cl/MnPY or SiO2/MnPY catalysts considerably more efficient than the corresponding, previously reported materials with mesoporous Santa Barbara Amorphous No 15 (SBA-15) silica. Among the materials studied, in situ derivatization of Mn(III) 2-N-pyridylporphyrin by covalent immobilization on Sil-Cl to yield Sil-Cl/MnP1 showed the best catalytic performance with high stability against oxidative destruction and reusability/recyclability.
- This article is part of the themed collection: New Talent: Americas


 Please wait while we load your content...
Please wait while we load your content...