Controlling the gate-sorption properties of solid solutions of Werner complexes by varying component ratios†
Abstract
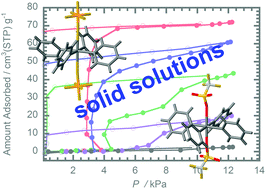
Methods relying on the use of solid solutions can be used to produce solid materials having finely controlled physical properties. In the current investigation, we utilized this protocol to prepare solid solutions derived from two different Werner complexes in order to assess the effects of component ratios on acetone vapor adsorption properties. For this purpose, microcrystalline solid solutions with the basic elemental composition α-[Cu(PF6)2(py)4]x[Cu(CF3SO3)2(py)4]1−x (x = 0.75 and 0.5) (α-PAC-2-PF6/CF3SO3(x = 0.75 and 0.5), py = pyridine) were prepared by hexane induced precipitation of acetone solutions of [Cu(PF6)2(py)4] (PAC-2-PF6) and [Cu(CF3SO3)2(py)4] (PAC-2-CF3SO3). The results of acetone sorption isotherm measurements show that gate opening and closing pressures of the solid solutions are dependent on the composition ratios of PAC-2-CF3SO3 and PAC-2-PF6. Specifically, an increase in CF3SO3− anion content induces weakening of the interactions with acetone as a consequence of expansion of lattice constants and also strengthening host–host interactions. These effects cause an increase in gate opening and closing pressures.


 Please wait while we load your content...
Please wait while we load your content...