Facile addition of E–H bonds to a dicarbondiphosphide†
Abstract
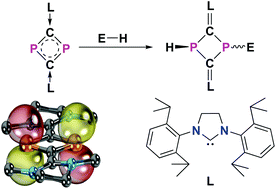
Charge transfer at P atoms in an N-heterocyclic carbene stabilized 6π-electron aromatic dicarbondiphosphide 1 has been observed upon interaction with a variety of small molecule substrates that feature a polar E–H bond (E = C, N, and O). As a result, these P atoms exhibit fleeting nucleophilicity and electrophilicity behaving as potential independent active sites, which effect the spontaneous addition of E–H bonds leading to a series of unsymmetrical 1,3-diphosphetane frameworks bearing P–C (2), P–N (6 and 7), and P–O bonds (8b, 8c, and 9). In addition, 1,3-diphosphetanium salts have been achieved when X-type substituents are weakly coordinating (i.e. [(CN)2CH]− (3), I− (4), and [(PhOH)2PhO]− (10)). The mechanisms of such transformations are investigated using density functional theory calculations.


 Please wait while we load your content...
Please wait while we load your content...