1,2-Insertion reactions of alkynes into Ge–C bonds of arylbromogermylene†
Abstract
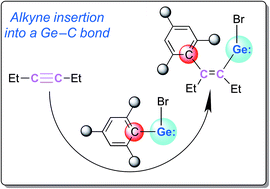
1,2-Insertion reactions of alkynes into the Ge–C bonds in dibromodigermenes afford stable crystalline bromovinylgermylenes. In contrast to previously reported Lewis-base-supported vinylgermylenes, the bromovinylgermylene obtained from reaction of the bromogermylene with 3-hexyne via such an 1,2-insertion is a donor-free monomer. A feasible reaction mechanism, proposed on the basis of the observed experimental results in combination with theoretical calculations, suggests that the [1+2]-cycloadduct and the insertion product are the kinetic and thermodynamic product, respectively.


 Please wait while we load your content...
Please wait while we load your content...