Na4Ni3P4O15–Ni(OH)2 core–shell nanoparticles as hybrid electrocatalysts for the oxygen evolution reaction in alkaline electrolytes†
Abstract
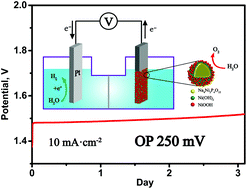
There is wide interest in developing efficient, robust and low-cost electrode materials for the electrolysis of water to produce clean hydrogen fuel. It is especially important to improve the performance and durability of electrocatalysts for the OER. Here we have shown that the transformation of nanoparticle (n-NNP) and crystalline (c-NNP) forms of mixed phosphate Na4Ni3(PO4)2P2O7 in highly alkaline solutions occurs along various routes and provokes the generation of 2D Ni(OH)2 nanosheets or stable core(phosphate)–shell(Ni(OH)2) particles, respectively. In both cases, in the carbon matrix (through chemical and electrochemical conversion of phosphate in situ during electrolysis in a 6 M KOH or NaOH solution) stable OER electrocatalysts with low overpotentials of 250–290 mV at a current density of 10 mA cm−2 were obtained. The best candidate for the OER process is core–shell particles, which maintain overpotentials of around 250 mV in 6 M KOH for more than 3 days. The activity enhancement can be attributed to the formation of abundant NiOOH nanoparticles on the shell surface due to improved lattice matching. This report discusses future prospects for the creation of core–shell particles to reduce the overpotential of durable electrocatalysts for the OER.


 Please wait while we load your content...
Please wait while we load your content...