The formation of PuSiO4 under hydrothermal conditions†
Abstract
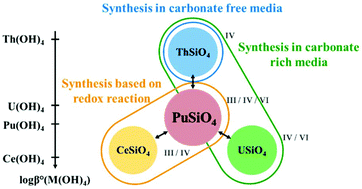
Attempts to synthesize plutonium(IV) silicate, PuSiO4, have been made on the basis of results recently reported in the literature for CeSiO4, ThSiO4, and USiO4 under hydrothermal conditions. Although it was not possible to prepare PuSiO4via applying the conditions reported for thorium and uranium, an efficient method of PuSiO4 synthesis was established by applying the conditions optimized for the CeSiO4 system. This method was based on the slow oxidation of plutonium(III) silicate reactants under hydrothermal conditions at 150 °C in hydrochloric acid (pH = 3–4). These results shed new light on the potential behavior of plutonium in reductive environments, highlighting the representative nature of cerium surrogates when studying plutonium under such conditions and providing some important pieces of information regarding plutonium chemistry in silicate solutions.


 Please wait while we load your content...
Please wait while we load your content...