Oxidative addition of activated aryl-carboxylates to Pd(0): divergent reactivity dependant on temperature and structure†
Abstract
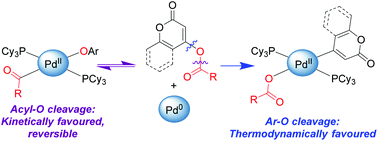
With the exception of activated sulfonate esters, oxidative addition of Ar–O bonds to Pd(0) complexes is extremely rare. This has led to a general perception that Pd-catalyzed cross-coupling is not feasible with O-based electrophiles such as aryl esters. We report that pyrone and coumarin esters do undergo oxidative addition to Pd(PCy3)2, with Pd insertion into either the acyl–O or Ar–O bond. Addition of the acyl–O bond to Pd(0) is kinetically favoured and reversible, while addition of the Ar–O bond is thermodynamically favoured. Using a larger and more electron-rich pivalate derivative disfavours acyl–O cleavage, enabling selective oxidative addition of the Ar–O bond and corresponding catalytic cross-coupling.
- This article is part of the themed collection: New Talent: Americas


 Please wait while we load your content...
Please wait while we load your content...