Pyridylamido hafnium complexes with a silylene bridge: synthesis and olefin polymerization†
Abstract
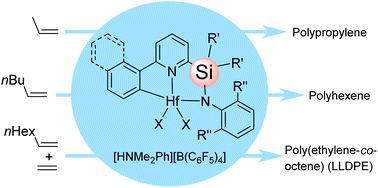
The synthesis and characterisation of six novel Cs-symmetric pyridylamido hafnium complexes with a silylene bridge of the type [ArPy(R2Si)NAr′]HfAlk2 are reported. Four complexes have been structurally characterised using single crystal X-ray diffraction. Appreciable differences between the solid state structures of these complexes and the pyridylamido hafnium complexes with a CRR′ bridge were noted. Reactions with B(C6F5)3, [Ph3C][B(C6F5)4] and [HMe2NPh][B(C6F5)4] yielded active catalysts for the homopolymerisations of propene and 1-hexene and ethene/1-octene copolymerization. In spite of the Cs-symmetry of the precatalysts, isotactically enriched polypropylene and poly(1-hexene) were obtained. The fact that the mechanism of the catalyst activation includes the insertion of alkene into the Hf-CAr bond was demonstrated. It was found that the structures of Ar and the R2Si bridge influence the activity, molecular weight capability and 1-octene affinity of the catalysts.


 Please wait while we load your content...
Please wait while we load your content...