One pot solvent assisted syntheses of Ag3SbS3 nanocrystals and exploring their phase dependent electrochemical behavior toward oxygen reduction reaction and visible light induced methanol oxidation reaction†
Abstract
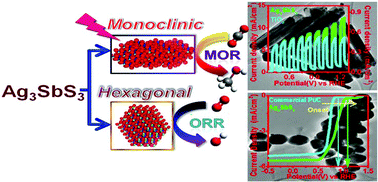
A huge variety of silver based ternary sulfide semiconductors (SCs) have been considered for the sustainable advancement of renewable energy sources. Herein, we have synthesized two important classes of newly emerging semiconductor nanocrystals (NCs) Ag3SbS3 (SAS), i.e. hexagonal and monoclinic by simply tuning the solvent polarity, of which the second one has been synthesized in a phase pure NC for the first time by the thermal decomposition of silver and antimony based dithiocarbamate (∼N-CS2-M) complexes. Interestingly, these two systems exhibit two different semiconducting (SC) properties and band gaps; hexagonal SAS has a p type (Eg ∼ 1.65 eV) whereas monoclinic SAS has an n type (Eg ∼ 2.1 eV) character. For the first time ever we have designed a reducing working electrode (i.e. cathode) by modifying the rotating disc electrode (RDE) with hexagonal SAS that exhibits excellent electrochemical oxygen reduction reaction (ORR) activity (Eonset = 1.09 V vs. RHE and average number of electron transfer: 3.89) comparable to that of the highly expensive Pt/C (Eonset = 0.88 V vs. RHE and average number of electron transfer: 3.92). Density functional theory (DFT) investigation confirms the corroborations of experimental data with theoretical implications. In addition, the electrode fabricated from monoclinic SAS acts as an efficient photoanode which exhibits higher photoelectrochemical (PEC) methanol oxidation reaction (MOR) activity under illumination in alkaline medium compared to that of standard TiO2 grown on an indium tin oxide (ITO) coated glass slide. On illumination, the relative photocurrent density at the onset potential has been obtained to be 845 which is a very significant experimental output with respect to any other TiO2 or Pt@TiO2 based photocatalysts for this application. The physicochemical stability and reusability of both materials were supported by 50 hours of extended electrochemical chronoamperometric measurements and powder XRD and the TEM analyses after electrocatalysis. This study explores a possible pathway for designing simple and less expensive but catalytically efficient silver based ternary sulfide NC systems for developing an SC material to reduce the energy crisis in the near future.


 Please wait while we load your content...
Please wait while we load your content...