A hemilabile manganese(i)–phenol complex and its coordination induced O–H bond weakening†
Abstract
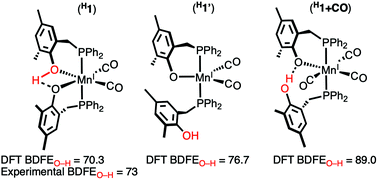
The known compound K[(PO)2Mn(CO)2] (PO = 2-((diphenylphosphino)methyl)-4,6-dimethylphenolate) (K[1]) was protonated to form the new Mn(I) complex (HPO)(PO)Mn(CO)2 (H1) and was determined to have a pKa approximately equal to tetramethylguanidine (TMG). The reduction potential of K[1] was determined to be −0.58 V vs. Fc/Fc+ in MeCN and allowed for an estimation of an experimental O–H bond dissociation free energy (BDFEO–H) of 73 kcal mol−1 according to the Bordwell equation. This value is in good agreement with a corrected DFT computed BDFEO–H of 68.0 kcal mol−1 (70.3 kcal mol−1 for intramolecular H-bonded isomer). The coordination of the protonated O-atom in the solid-state H1 was confirmed using FTIR spectroscopy and X-ray crystallography. The phenol moiety is hemilabile as evident from computation and experimental results. For instance, dissociation of the protonated O-atom in H1 is endergonic by only a few kcal mol−1 (DFT). Furthermore, [1]− and other Mn(I) compounds coordinated to PO and/or HPO do not react with MeCN, but H1 reacts with MeCN to form H1+MeCN. Experimental evidence for the solution-bound O-atoms of H1 was obtained from 1H NMR and UV-vis spectroscopy and by comparing the electronic spectra of bona fide 16-e− Mn(I) complexes such as [{PNP}Mn(CO)2] (PNP = −N{CH2CH2(PiPr2)}2) and [(Me3SiOP)(PO)Mn(CO)2] (Me3Si1). Compound H1 is only meta-stable (t1/2 0.5–1 day) and decomposes into products consistent with homolytic O–H bond cleavage. For instance, treatment of H1 with TEMPO resulted in formation of TEMPOH, free ligand, and [MnII{(PO)2Mn(CO)2}2]. Together with the experimental and calculated weakened BDFEO–H, these data provide strong evidence for the coordination and hemilability of the protonated O-atom in H1 and represents the first example of the phenolic Mn(I)–O linkage and a rare example of a “soft-homolysis” intermediate in the bond-weakening catalysis paradigm.
- This article is part of the themed collection: New Talent: Americas


 Please wait while we load your content...
Please wait while we load your content...