The Pd(0) and Pd(ii) cocatalyzed isomerization of alkynyl epoxides to furans: a mechanistic investigation using DFT calculations†
Abstract
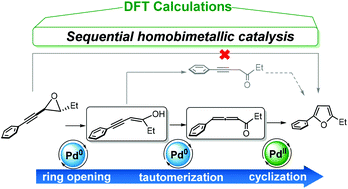
The conversion of alkynyl epoxides to furans is an unusual tandem catalytic process in which two different oxidation states of palladium are employed. In this study, we used density functional theory calculations to establish the mechanistic details of the catalytic cycles for all the individual processes in this conversion. The results showed that the use of Pd(0) or Pd(II) alone as the catalyst leads to high reaction barriers. This finding is consistent with experimental observations of low furan yields and the need for high temperatures in the presence of either catalyst alone. However, a combination of Pd(0) and Pd(II) lowers the reaction barriers considerably. Our key finding is that the reaction pathway involves epoxide ring opening catalyzed by Pd(0), followed by tautomerization of an enol to generate an allenyl ketone in conjunction with Pd(0), with a subsequent Pd(II)-catalyzed cyclization to yield the furan.


 Please wait while we load your content...
Please wait while we load your content...