Fluorometric detection of iodine by MIL-53(Al)-TDC†
Abstract
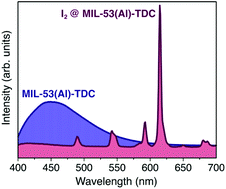
The fluorescent properties of MIL-53(Al)-TDC are drastically changed due to the presence of iodine, even in small quantities, as a result of an energy transfer process from the host material (MIL-53(Al)-TDC) to the guest molecule (I2). While MIL-53(Al)-TDC's emission spectrum shows a weak and broad band, after I2 adsorption, it exhibits well-resolved and long-lasting emission lines, which could be exploited for iodine detection. Density Functional Theory periodical calculations demonstrated that in the most stable MIL-53(Al)-TDC⋯I2 configuration, the I2 molecule is bonded mainly by an O–H⋯I hydrogen bond. The QTAIM showed that other non-covalent interactions also provided stability to MIL-53(Al)-TDC⋯I2. The electrostatic potential analysis indicated that the I2 molecule adsorption occurs by a combination of specific interactions with a strong electrostatic contribution and weak interactions. These results postulate fluorescent MIL-53(Al)-TDC as an efficient I2 detector (potentially for radioactive I2), using a simple fluorimetric test.
- This article is part of the themed collection: Celebrating recent chemical science in Mexico


 Please wait while we load your content...
Please wait while we load your content...