Enhancing the energy barrier by replacing the counterions in two holmium(iii)-pentagonal bipyramidal single-ion magnets†
Abstract
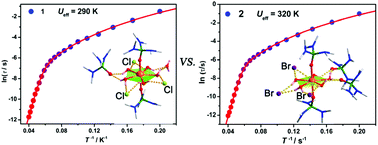
Based on a phosphine oxide ligand, HMPA (hexamethylphosphoric triamide), two mononuclear HoIII-pentagonal bipyramidal complexes were synthesized with the formulas [Ho(HMPA)2(H2O)5]2Cl6·2HMPA·2H2O (1) and [Ho(HMPA)2(H2O)5]Br3·2HMPA (2). Single-crystal X-ray diffraction results show that all HoIII ions in both the two complexes are hepta-coordinated and are located in pentagonal bipyramidal {HoO7} coordination polyhedrons constructed by two axial HMPA ligands and five equatorial water molecules. However, due to the employment of different halide ions as counterions, the second coordination sphere surrounding each [Ho(HMPA)2(H2O)5]3+ moiety is different in the two complexes: in 1, three Cl− ions, one water molecule and one HMPA ligand; in 2, three Br− ions and two HMPA ligands. Ac magnetic susceptibilities under zero dc field show that both the two complexes are single-ion magnets with effective energy barriers of 290 K and 320 K for 1 and 2, respectively. Compared with 1, the enhancement in the energy barrier of 2 is believed to be induced mainly by the change in the second coordination sphere rather than the minor differences in the {HoO7} polyhedrons.


 Please wait while we load your content...
Please wait while we load your content...