Redox-active ligand assisted electrocatalytic water oxidation by a mononuclear cobalt complex†
Abstract
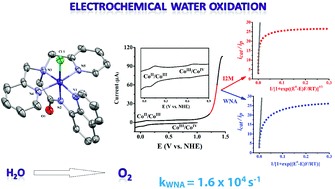
In this report, the synthesis, characterization and electrocatalytic oxidation of water by a mononuclear cobalt(III) complex, [CoIII(dpaq)(Cl)]Cl (1) featuring a redox-active pentadentate amidate ligand (H-dpaq = 2-[bis(pyridin-2-ylmethyl)]amino-N-quinolin-8-yl-acetamidate) is reported. Complex 1 has been found to be a stable and homogeneous water oxidation catalyst (WOC) in 0.1 M phosphate buffer (pH 8.0). A series of experiments (rinse test, SEM, EDX spectroscopy) confirm that this complex acts as a molecular electrocatalyst, and not a precursor of CoOx. The electrocatalytic water oxidation proceeds with high faradaic efficiency (81%) and fast rate (85 s−1). Analysis of the electrochemical reaction kinetics by foot-of-the-wave (FOWA) methodology reveals a high turnover frequency of 1.6 × 104 s−1 which is comparable to the best performing Ru-based WOCs.


 Please wait while we load your content...
Please wait while we load your content...