Neutral binary chalcogen–nitrogen and ternary S,N,P molecules: new structures, bonding insights and potential applications
Abstract
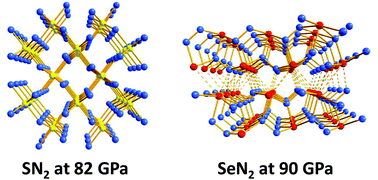
Early theoretical and experimental investigations of inorganic sulfur–nitrogen compounds were dominated by (a) assessments of the purported aromatic character of cyclic, binary S,N molecules and ions, (b) the unpredictable reactions of the fascinating cage compound S4N4, and (c) the unique structure and properties of the conducting polymer (SN)x. In the last few years, in addition to unexpected developments in the chemistry of well-known sulfur nitrides, the emphasis of these studies has changed to include nitrogen-rich species formed under high pressures, as well as the selenium analogues of well-known S,N compounds. Novel applications have been established or predicted for many binary S/Se,N molecules, including their use for fingerprint detection, in optoelectronic devices, as high energy-density compounds or as hydrogen-storage materials. The purpose of this perspective is to evaluate critically these new aspects of the chemistry of neutral, binary chalcogen–nitrogen molecules and to suggest experimental approaches to the synthesis of target compounds. Recently identified ternary S,N,P compounds will also be considered in light of their isoelectronic relationship with binary S,N cations.
- This article is part of the themed collections: 2020 Frontier and Perspective articles and Inorganic chemistry of the p-block elements


 Please wait while we load your content...
Please wait while we load your content...