Boronic, diboronic and boric acid esters of 1,8-naphthalenediol – synthesis, structure and formation of boronium salts†
Abstract
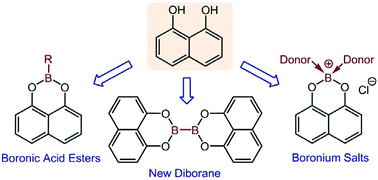
The 1,8-naphthalenediolate [1,8-O2C10H8] supported boronic and boric acid esters of general formula X-B(1,8-O2C10H8), where X = C6H5 (1a), C6F5 (2a), 3,4,5-F3-C6H2 (3a), 2,4,6-F3-C6H2 (4a), 2,6-F2-C6H3 (5a), 2,6-Cl2-C6H3 (6a), 2,4,6-Me3-C6H2 (7a), 2,6-(MeO)3-C6H3 (8a), Bun (9a), MeO (10a), OH (11a) and Cl (13a), were synthesized, NMR spectroscopically characterized, and the solid-state structures of 1a–5a, 8a and 10a determined by X-ray crystallography. The acceptor numbers of 1a–7a and 13a were determined and found to be similar to their catecholate analogues, R-Bcat, indicating similar Lewis acidities of these two classes of boronic acid esters. The reaction of B2(NMe2)4 with 1,8-naphthalenediol, followed by addition of HCl furnished the diboronic acid ester B2(1,8-O2C10H8)4 (16a) in ca. 70% yield. Cl-B(1,8-O2C10H8) (13a) was shown to react with O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PEt3, DMAP, 1,10-phenanthroline and 2,2′-bipyridine, resp., to give the boronium salts [(Et3P
PEt3, DMAP, 1,10-phenanthroline and 2,2′-bipyridine, resp., to give the boronium salts [(Et3P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)2B(1,8-O2C10H8)]Cl (18a), [(DMAP)2B(1,8-O2C10H8)]Cl (22a), [(2,2′-bipyridine)B(1,8-O2C10H8)]Cl (23a) and [(1,10-phenanthroline)B(1,8-O2C10H8)]Cl (24a), which were characterized by NMR spectroscopy and X-ray crystallography.
O)2B(1,8-O2C10H8)]Cl (18a), [(DMAP)2B(1,8-O2C10H8)]Cl (22a), [(2,2′-bipyridine)B(1,8-O2C10H8)]Cl (23a) and [(1,10-phenanthroline)B(1,8-O2C10H8)]Cl (24a), which were characterized by NMR spectroscopy and X-ray crystallography.


 Please wait while we load your content...
Please wait while we load your content...