Identification of key functionalization species in the Cp*Ir(iii)-catalyzed-ortho halogenation of benzamides†
Abstract
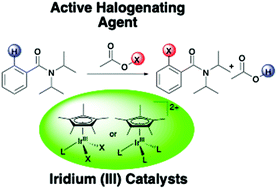
Cp*Ir(III) complexes have been shown to be effective for the halogenation of N,N-diisopropylbenzamides with N-halosuccinimide as a suitable halogen source. The optimized conditions for the iodination reaction consist of 0.5 mol% [Cp*IrCl2]2 in 1,2-dichloroethane at 60 °C for 1 h to form a variety of iodinated benzamides in high yields. Increasing the catalyst loading to 6 mol% and the time to 4 h enabled the bromination reaction of the same substrates. Reactivity was not observed for the chlorination of these substrates. A variety of functional groups on the para-position of the benzamide were well tolerated. Kinetic studies showed the reaction dependence is first order in iridium, positive order in benzamide, and zero order in N-iodosuccinimide. A KIE of 2.5 was obtained from an independent H/D kinetic isotope effect study. Computational studies (DFT-BP3PW91) indicate that a CMD mechanism is more likely than an oxidative addition pathway for the C–H bond activation step. The calculated functionalization step involves an Ir(V) species that is the result of oxidative addition of acetate hypoiodite that is generated in situ from N-iodosuccinimide and acetic acid.
- This article is part of the themed collection: New Talent: Americas


 Please wait while we load your content...
Please wait while we load your content...