Formation mechanism of an Al13 Keggin cluster in hydrated layered polysilicates†
Abstract
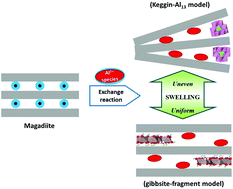
An Al13 ε-Keggin cluster, AlO4Al12(OH)24(H2O)127+, is a predominant intermediate during the hydrolysis and polymerization of aluminum as well as a highly toxic substance to plants and fishes. However, no one could clearly explain why and how a cage-like Al13 ε-Keggin cluster is formed even though it could be readily synthesized by the forced hydrolysis of Al3+. We found that the Al13 ε-Keggin cluster was spontaneously formed not in monocrystalline octosilicate but in polycrystalline magadiite by the cation-exchange reaction with unhydrolyzed Al3+. Furthermore, the Al13 ε-Keggin cluster was hardly detected in disaggregated magadiite crystals whose morphology was changed into monocrystalline crystals like octosilicate. Our findings prove that Al13 formation is necessary to relieve localized inhomogeneity and rationalize that Al13 is formed by the simultaneous co-assembly of four planar trimers and one octahedral monomer. In addition, the spontaneous formation of Al13 in heterogeneous systems could be a vital clue to its evaluation in soils and sediments.


 Please wait while we load your content...
Please wait while we load your content...