Synthetic investigations of low-coordinate (N-phosphino-amidinate) nickel chemistry: agostic alkyl complexes and benzene insertion into Ni–H†
Abstract
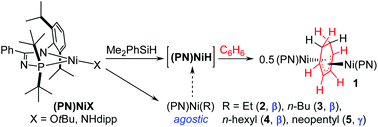
Treatment of (PN)NiX (X = NHdipp or OtBu; PN = N-phosphinoamidinate ligand) with Me2PhSiH in benzene solvent afforded the crystallographically characterized, antifacial-coordinated, dinuclear species 1, the formation of which corresponds to the hitherto unknown net Ni–H addition of two equivalents of the putative (PN)NiH intermediate across C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C units within a single benzene molecule. Computational analysis supports the view of 1 as being comprised of two cationic (PN)NiII fragments ligated by a substituted butadiene dianion μ2–η3:η3-C6H82− bridging group. Also described is the formation and characterization of three-coordinate (PN)Ni(alkyl) complexes stabilized by β-agostic (alkyl = Et, 2; n-Bu, 3; n-hexyl, 4) or γ-agostic (alkyl = neopentyl, 5) interactions, and our efforts to employ 2 and 3 as synthons for the generation of (PN)NiHvia β-hydride elimination. Notably, compound 5 represents both the first crystallographically characterized three-coordinate Ni-alkyl complex featuring a heterobidentate ligation, and the first neutral γ-agostic NiII-alkyl complex.
C units within a single benzene molecule. Computational analysis supports the view of 1 as being comprised of two cationic (PN)NiII fragments ligated by a substituted butadiene dianion μ2–η3:η3-C6H82− bridging group. Also described is the formation and characterization of three-coordinate (PN)Ni(alkyl) complexes stabilized by β-agostic (alkyl = Et, 2; n-Bu, 3; n-hexyl, 4) or γ-agostic (alkyl = neopentyl, 5) interactions, and our efforts to employ 2 and 3 as synthons for the generation of (PN)NiHvia β-hydride elimination. Notably, compound 5 represents both the first crystallographically characterized three-coordinate Ni-alkyl complex featuring a heterobidentate ligation, and the first neutral γ-agostic NiII-alkyl complex.


 Please wait while we load your content...
Please wait while we load your content...