Catalytic dioxygen reduction mediated by a tetranuclear cobalt complex supported on a stannoxane core†
Abstract
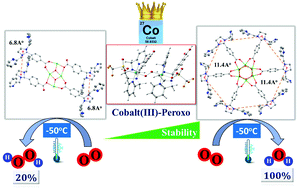
The synthesis, spectroscopic characterization (infrared, electron paramagnetic resonance and X-ray absorption spectroscopies) and density functional theoretical calculations of a tetranuclear cobalt complex Co4L1 involving a nonheme ligand system, L1, supported on a stannoxane core are reported. Co4L1, similar to the previously reported hexanuclear cobalt complex Co6L2, shows a unique ability to catalyze dioxygen (O2) reduction, where product selectivity can be changed from a preferential 4e−/4H+ dioxygen-reduction (to water) to a 2e−/2H+ process (to hydrogen peroxide) only by increasing the temperature from −50 to 30 °C. Detailed mechanistic insights were obtained on the basis of kinetic studies on the overall catalytic reaction as well as by low-temperature spectroscopic (UV-Vis, resonance Raman and X-ray absorption spectroscopies) trapping of the end-on μ-1,2-peroxodicobalt(III) intermediate 1. The Co4L1- and Co6L2-mediated O2-reduction reactions exhibit different reaction kinetics, and yield different ratios of the 2e−/2H+ and 4e−/4H+ products at −50 °C, which can be attributed to the different stabilities of the μ-1,2-peroxodicobalt(III) intermediates formed upon dioxygen activation in the two cases. The deep mechanistic insights into the transition-metal mediated dioxygen reduction process that are obtained from the present study should serve as useful and broadly applicable principles for future design of more efficient catalysts in fuel cells.
- This article is part of the themed collection: Inorganic Reaction Mechanisms


 Please wait while we load your content...
Please wait while we load your content...