Dehydrogenation of amines in aryl-amine functionalized pincer-like nitrogen-donor redox non-innocent ligands via ligand reduction on a Ni(ii) template†‡
Abstract
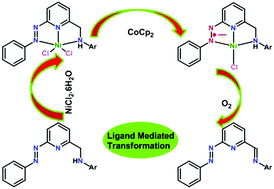
We have synthesized a series of new redox non-innocent azo aromatic pincer-like ligands: 2-(phenylazo)-6-(arylaminomethyl)pyridine (HLa–c: HLa = 2-(phenylazo)-6-(2,6-diisopropylphenylaminomethyl)pyridine, HLb = 2-(phenylazo)-6-(2,6-dimethylphenylaminomethyl)pyridine, and HLc = 2-(phenylazo)-6-(phenylaminomethyl)pyridine), in which one side arm is an arylaminomethyl moiety and the other arm is a 2-phenylazo moiety. Nickel(II) complexes, 1–3, of these ligands HLa–c were synthesized in good yield (approximately 70%) by the reaction of ligands : (NiCl2·6H2O) in a 1 : 1 molar ratio in methanol. The amine donor in each of the ligands HLa–c binds to the Ni(II) centre without deprotonation. In the solid state, complex 3 is a dimer; in solution it exists as monomer 3a. The reduction of acetonitrile solutions of each of the complexes 1, 2 and 3a, separately, with cobaltocene (1 equivalent), followed by exposure of the solution to air, resulted in the formation of new complexes 7, 8 and 9, respectively. Novel free ligands Lx and Ly have also been isolated, in addition to complexes 7 and 8, from the reaction of complexes 1 and 2, respectively. Complexes 7–9 and free ligands Lx and Ly have been formed via a dehydrogenation reaction of the arylaminomethyl side arm. The mechanism of the reaction was thoroughly investigated using a series of studies, including cyclic voltammetry, EPR, and UV-Vis spectral studies and density functional theory (DFT) calculations. The results of these studies suggest a mechanism initiated by ligand reduction followed by dioxygen activation. A Cl−/I− scrambling experiment revealed that the dissociation of the chloride ligand(s) was associated with the one-electron reduction of the ligand (azo moiety) in each of the complexes 1, 2 and 3a. The dissociated chloride ligand(s) were reassociated with the metal following the dehydrogenation reaction to yield the final products. All of the newly synthesized compounds were fully characterized using a variety of physicochemical techniques. Single-crystal X-ray structures of the representative compounds were determined to confirm the identities of the synthesized molecules.


 Please wait while we load your content...
Please wait while we load your content...